With an aging pet population, cancer became the number one cause of morbidity and mortality in dogs and cats. Treatment of cancer in pets is very similar to humans: surgery, chemotherapy, radiation, and immunotherapy are the most common, but the efficacy is very limited. Tumor gene therapy for pets is expected as a novel drastic method for tumor therapy as well as for human. However, only a few gene medicine has been approved for pets or human.
This project aims to develop a novel highly effective gene medicine for animal tumor therapy.
- [I] Clarification of problems
- <Viral Gene Therapy -limit of application->
In order to introduce the therapeutic gene into the target cells, a carrier called a "vector" is generally required. Several kinds of virus vectors have been developed and used as a delivery vehicle for human clinical trials of gene therapy. They showed fairly high clinical effects in some cases, and some have already been approved for commercial use.
However, viral vectors still have risks such as random recombination and immunogenicity, and indeed, some viral gene therapy experiments have produced mortal side effects.
Moreover, according to the Cartagena Protocol on Biosafety, treatment of the animals which were injected with a genetically modified virus is strictly limited. It would make it difficult to make general use of the viral medicines.
- <Problem in Non-Viral Gene Therapy>
Thus, safer alternative nonviral vectors have been explored as synthetic gene delivery systems, and certain polycations or cationic lipids were found to be able to mediate gene transfection. Those non-viral vectors electrostatically associate with the DNA molecule, and form small complex particles. When the DNA complex is added onto the cultured cells, they can be taken up by the cells, and effectively express the encoded gene.
Although certain cationic agents have already achieved the high gene transfection efficiency on the cells, the gene expression in the living body by those artificial vectors is strictly limited. The application of the artificial vectors to clinical use is, thus, still particularly challenging.
- [II] Analysis of the Problems
Why the synthetic vectors failed in high gene expression in the body?
The low expression efficiency of the non-viral vectors in the body should be attributed to low delivery efficiency to the target cells. Major obstacles to the efficient delivery are thought to be
(1) non-specific interaction with bio-components, and (2) large size of the complex particles.
- [III] Solution Strategy and its Prospect
- (1) Non-specific interaction
Synthetic vectors, such as polycations or cationic lipids, electrostatically associate with the DNA molecule to form small complex particles. Since DNA is a relatively rigid molecule, excess cations are usually required to form a stable complex. Surface of the complexes are, thus, positively charged, and it invited a non-specific interaction in the body with blood calls, serum proteins and extracellular matrices. Shielding of the charge by grafting the neutral polymer onto the complex surface was reported [Ogris M, et al. Gene Ther. 1999;6:595]. It could restrain the undesirable interactions, and enhanced the duration in the blood stream. But the procedure of the chemical modification of the pre-formed particle is complicated, and often hard to reproduce. Pre-modification of the polycation by neutral polymers was also reported (Wolfert et al. Hum Gene Ther. 1996; 7:2123). However, arranging the polycation is also complex, and may cause the loss of transfection-mediating ability of the polycation.
Addition of polyanions to positively charged DNA/polycation complex seems to lead to a formation of the polyanion-coated negatively charged complex particles. But, common polyanions, such as heparin or polyacrylic acid, will disrupt DNA/polycation complex to release free DNA molecule. Whereas, we found that certain particular polyanions could be deposited onto the positively charged surface of DNA complex to form DNA/polycation/polyanion ternary structures without destroying them.
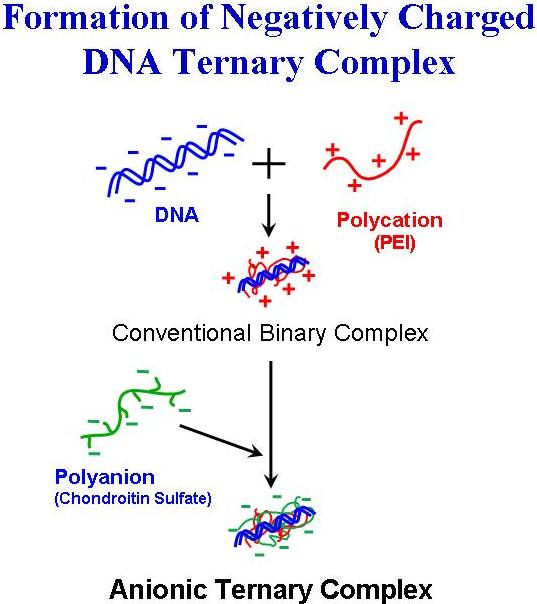
Addition of hyaluronic acid (HA), or chondroitin sulfate (CS) could recharge the DNA/polycation complex to negative, and effectively diminished the non-specific interaction with blood cells or other biocomponents.
The polyanion could play a role as not only protective coating, but as a ligand to target cells, and also a transcriptional enhancer.
- (2) Large size of the complex
Most serious problem remained unsolved was the difficulty in achieving and maintaining the small size of the DNA complexes. Small particles can only be obtained by mixing DNA with the polycation at very low concentration, though fairly high concentration is necessary to administer an adequate dose of DNA for gene transfection in the living body. Unfortunately, DNA/polycation complex will very readily adhere to each other to large aggregates. Condensation of the small DNA/polycation prepared at very low concentration, thus, always causes the aggregation and inactivation.
We found that HA- or CS-coated DNA complexes were very stably dispersed in the solution. And, under certain particular conditions, they could be freeze-dried without aggregation nor loss of activity. By mixing of DNA, polycation, and HA (or CS) at highly diluted concentration, followed by lyophilizing-rehydrating condensation, very fine DNA complex particles (< 70 nm) at high concentration (> 500 mg/ml) were finally achieved.
- [IV] Highly Effective Transfection System
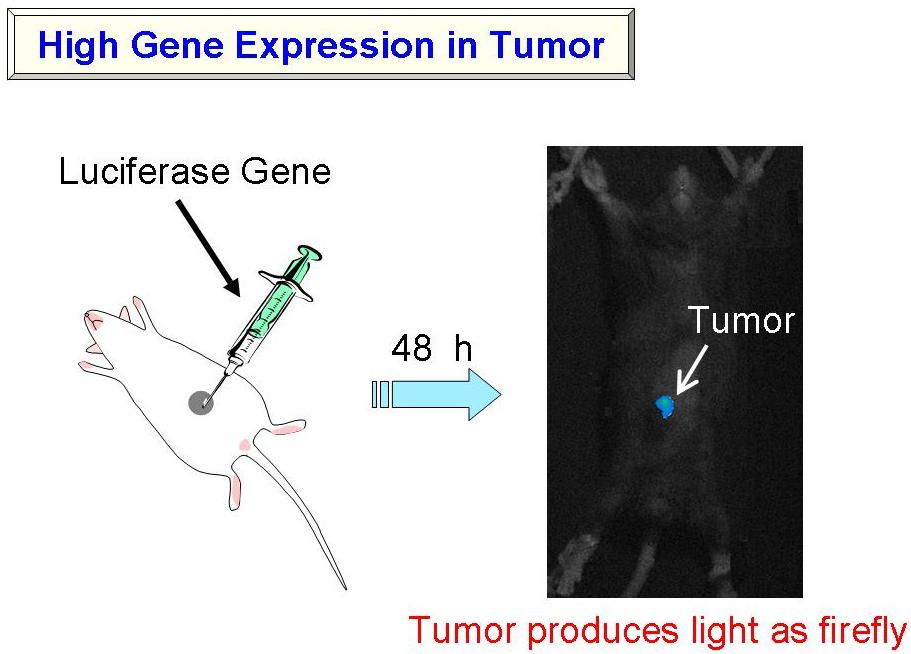
Make Tumor Glowing like a Firefly
- (1) Achievement of High Gene Expression in the Body
As mentioned above, we have attained the preparation of very small DNA complex suspension by preparing the complexes at highly diluted conditions, followed by lyophilization-condensation. Reporter-gene expression of the formulation was examined in tumor-bearing mice. The small complex consisting of DNA encoding luciferase, a firefly enzyme that catalyses the generation of light, was prepared, and administered into tumor-bearing mice. After intravenous- or intratumoral-injection, tumor produces strong light as firefly.
 Activate a Powerful Enemy Against Tumor Cells - Cytokine Transfection to Activate the Immune System -
Activate a Powerful Enemy Against Tumor Cells - Cytokine Transfection to Activate the Immune System -
- (2) High Efficacy in Tumor Therapy
The small complex was prepared with DNA encoding cytokine, an immune stimulating protein, and examined for the therapeutic efficacy in tumor-bearing mice. After five-times-injection of the complex, tumor completely disappeared in all the mice.

- (3) Genes for Higher Efficacy
Don't Let the Tumor Cells Escape - Marking the Tumor Cells by Tuberculosis Protein -
- <Novel strategy to overcome the immuno-escape of tumor>
Immune escape of the tumor cells is one of the main obstacles hindering the immunotherapy. We have developed a novel strategy to overcome the immuno-escape mechanism of the tumor cells. When a human is infected with a virus or bacteria, the viral- or bacterial antigenic proteins are expressed on the cell surface, and it would be recognized by antigen-presenting cells (APCc) as a "danger signal", and the immune systems would be stimulated.
We thought that not only the virus infection, but transfection of the virus- or bacteria-specific antigenic protein genes could also induce the pathogenic antigen presentation on the cell surfaces. Transfection with a pathogenic antigen gene into tumor cells, should, thus, be expected to lead to highly effective anti-tumor immune-enhancement. We examined several pathogenic protein genes, and certain myco-bacterium tuberculosis antigens are revealed to have high anti-tumor efficacy
Transfection of the tumor-bearing mice with these pathogenic genes showed as high anti-tumor activity as that with cytokine genes. Co-transfection of the pathogenic antigen-gene with cytokine-genes showed evidently superior anti-tumor therapeutic effect than either gene alone.
- [V] Animal Clinical Results
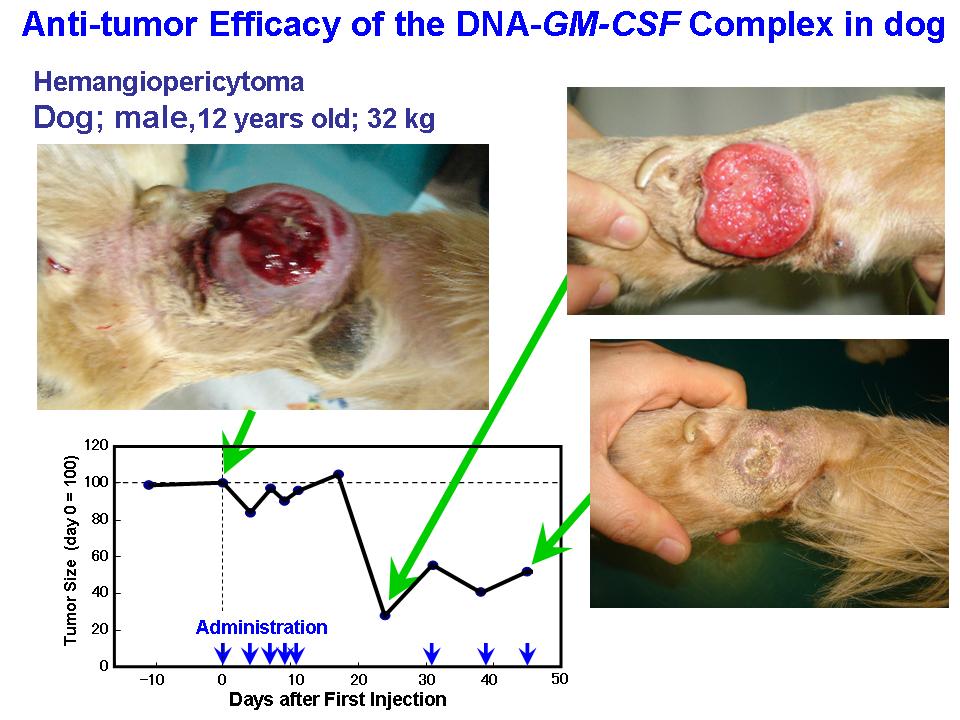
- <Animal clinical study of cytokine gene-transfection>
Efficacy and safety of the small complex was confirmed by studies on mice. Clinical effect of the complex comprising DNA coding GM-CSF was then examined on primary tumor in dogs, and cats. Those synthetic gene delivery systems showed remarkable anti-tumor efficacy on the animals.
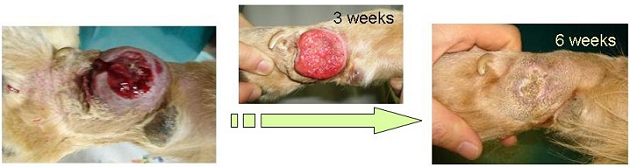
- <Animal clinical study of bacterial gene-transfection>
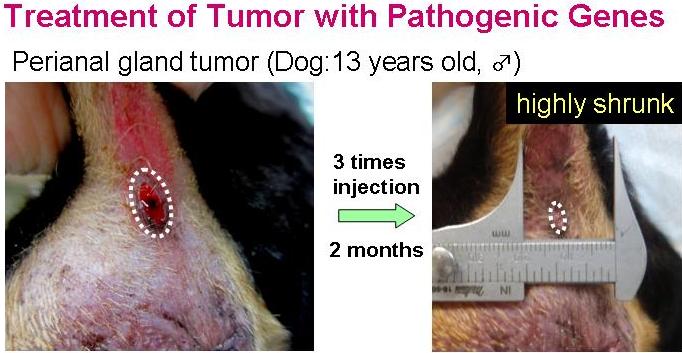
Efficacy of the transfection with the myco-bacterium tuberculosis antigen gene was examined on primary tumor in dogs, and cats. Those pathogenic genes also induced evident tumor regression in the animals.
We have developed a novel artificial transfection system comprising very small (<70 nm) DNA complex particles with negative surface charge. They could induce high extragene expression in tumor tissue.
Those negatively charged DNA complex showed much lower cytotoxicity and whole-body toxicity than the conventional positively charged complexes.
The small complex made of the DNA coding cytokine genes showed evident therapeutic effect not only in the mice, but on dogs and cats suffering from tumors.
Co-transfection of the bacterial antigen-gene with cytokine-genes was found to have superior anti-tumor therapeutic, and could induce remarkable tumor regression in dogs and cats.
The formulation is a white sponge, which can be
・easily re-hydrated to a solution by adding water
・stored at 4℃ for months without inactivation
It should be the only potential candidate of non-viral gene transfection system, to date.
The pet animal health care market is huge (North America: US$2.3 billion, Western Europe: US$962 million, and Australia: US$145 million), and still growing at a rate of 3.7% in 2010.
Besides, pet insurance penetration rates rising every year, and the sales of pet insurance in the United States totaled $303 million in 2009, up 16% from $262 million in 2008. It should assist the prevalence of animal tumor-treatment.
Animal anti-tumor medicine is a drug worth developing, now.
Contact Us
Return to top





