Research Subjects
- (1) Bioadhesive Gels for Hemostatics and Adhesion Barrier Devices
--Clinical Study as a Hemostatic Device--
- 1-1. Introduction
Protein-based devices are widely used for topical hemostasis. However, there still remains risks of virus- or BSE-infection. Polysaccharide-devices are also available on the market. But, they are sometimes reported to causes inflammation. They do not stick to bleeding tissue itself, and should be held firmly until hemostasis occurs. Ideal hemostatic material would be safe, easy to use, highly efficacious, fully absorbable, and inexpensive. Unfortunately, it does not exist.
- 1-2. Development of Novel Bioadhesive Gels for Hemostatic Device
a flexible transparent film, and a fluffy sponge, and a soft sheet, and a powder
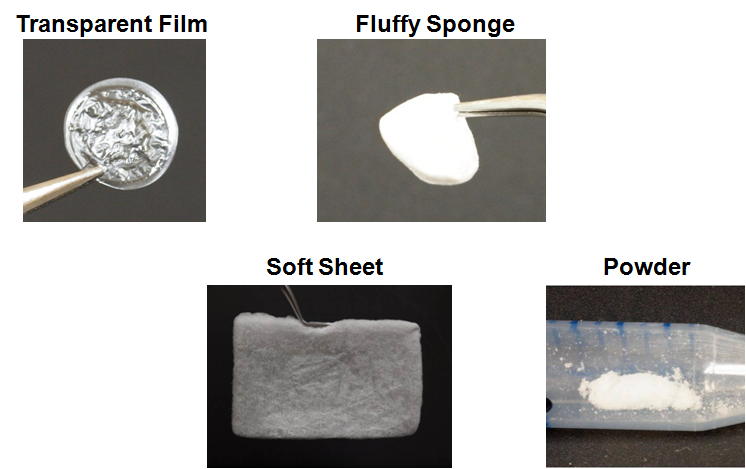
They swelled in water to a hydrogel.
On putting on the wet tissue, they would absorb water to a soft hydrogel,
and adhere to tissues.

They are highly safe:
All the ingredients have already been approved as pharmaceutical additives.
Fully degradable in body:
The gels will be slowly dissolved at pH 7.4.
- 1-3. Development of Hemostatic Film
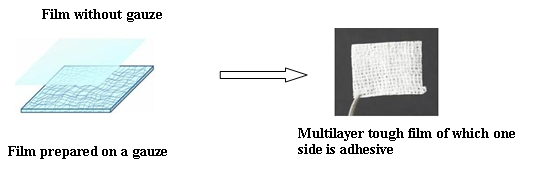
<Clinical Study>
Clinical Studies of the Film for Hemostasis after Blood Sampling or Intravenous Injection was Performed in the Patients Taking Anticoagulant Medicines.
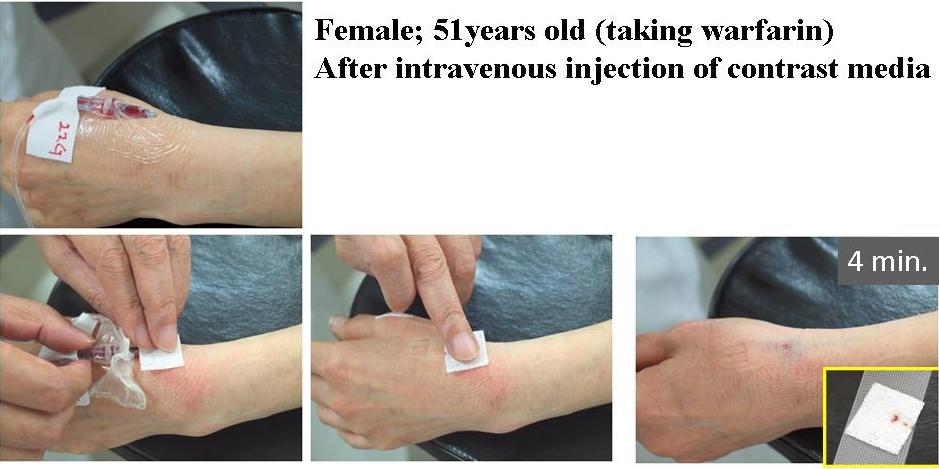
PAA/PVP film was placed on the bleeding site. It swelled soon to a soft gel, and adhered to skin. Bleeding was completely stopped very quickly, though the patient takes anticoagulant.
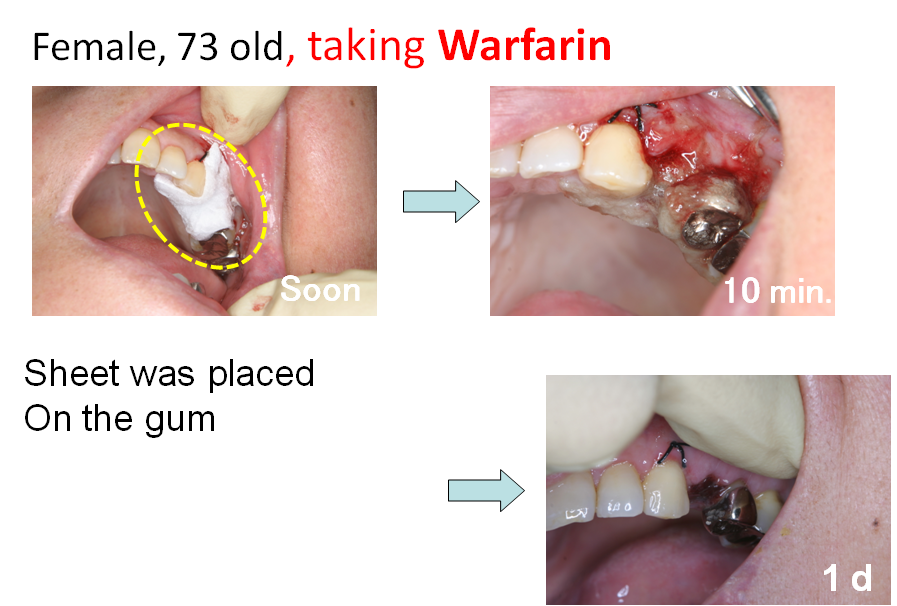
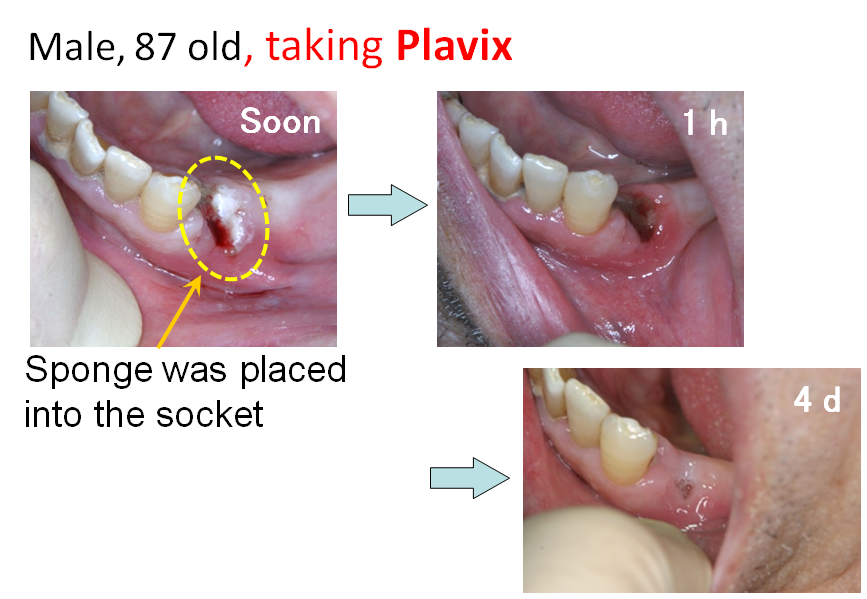
- 1-4. Development of Hemostatic Sponge
<Clinical Study>
Clinical studies of the sponge was carried out in dental surgery field. PAA/PVP sponge was placed in the socket after teeth extraction. It swelled to a soft gel, and filled the socket. Even when the patient takes anticoagulant, bleeding was efficiently stopped quickly. No adverse side effect was observed in the clinical study.
- 1-5. Effect of the Bioadhesive Gels as an Adhesion Barrier Devices
Possibility of the film or sponge as an adhesion barrier was then examined.
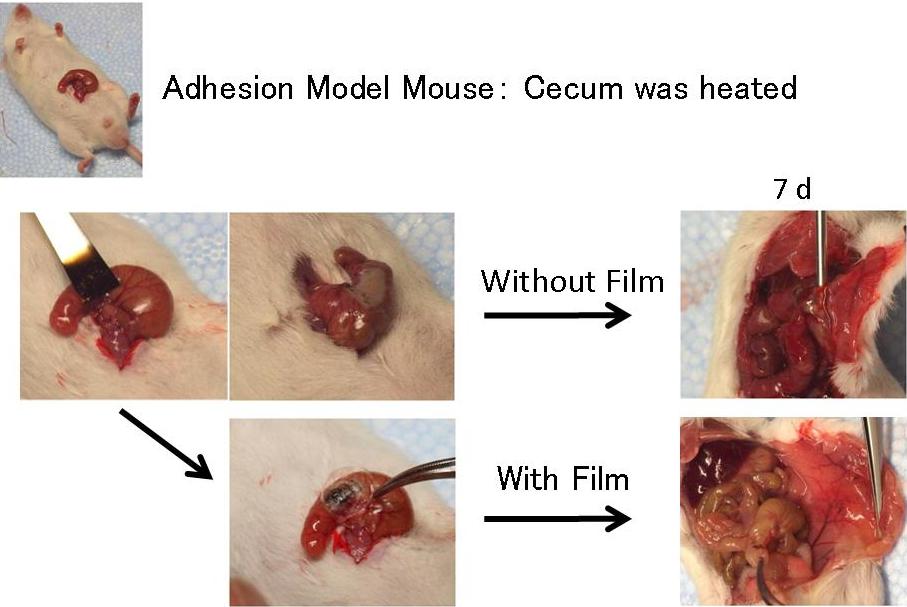
Without the film, the cecum stack firmly to peritoneum or other intestines, whereas the secum treated with the film did not adhere to other tissues.
- 2-1. Introduction
Major obstacles to the efficient in vivo transfection would be (1) the adverse interaction of the complex with biocomponents, and (2) too large size of the complex particles to be delivered to the target cells.
- 2-2. Development of Novel Efficient Synthetic Gene Delivery Systems
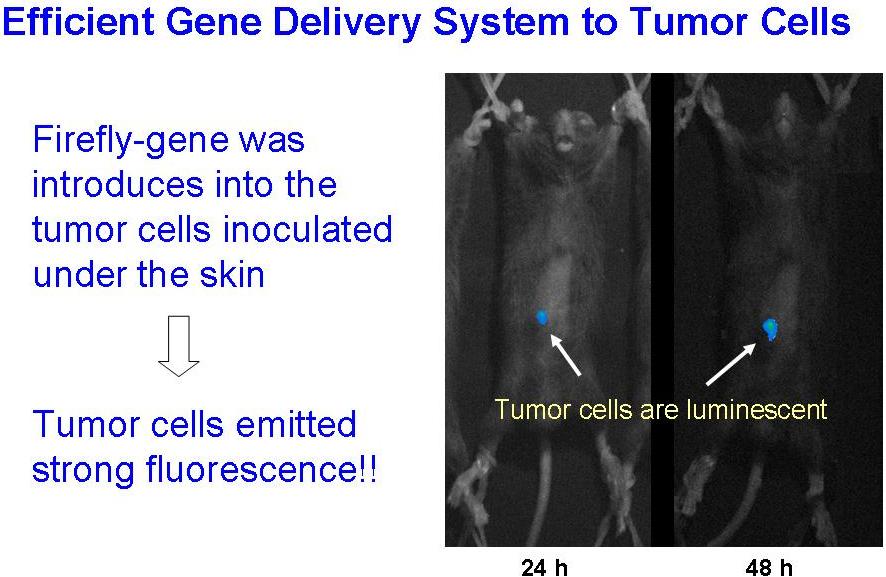
The polysaccharide-coating was also found to protect the DNA/PEI complexes against aggregation and inactivation through lyophilization-and-rehydration procedures. It allows us to prepare the concentrated very small DNA complex particles (< 70 nm) suspension by preparing the complexes at highly diluted conditions, followed by lyophilized-and-rehydrated to a small volume.
Those formulations achieved high reporter gene expression level in tumor after intravenous- or intratumoral-injection.
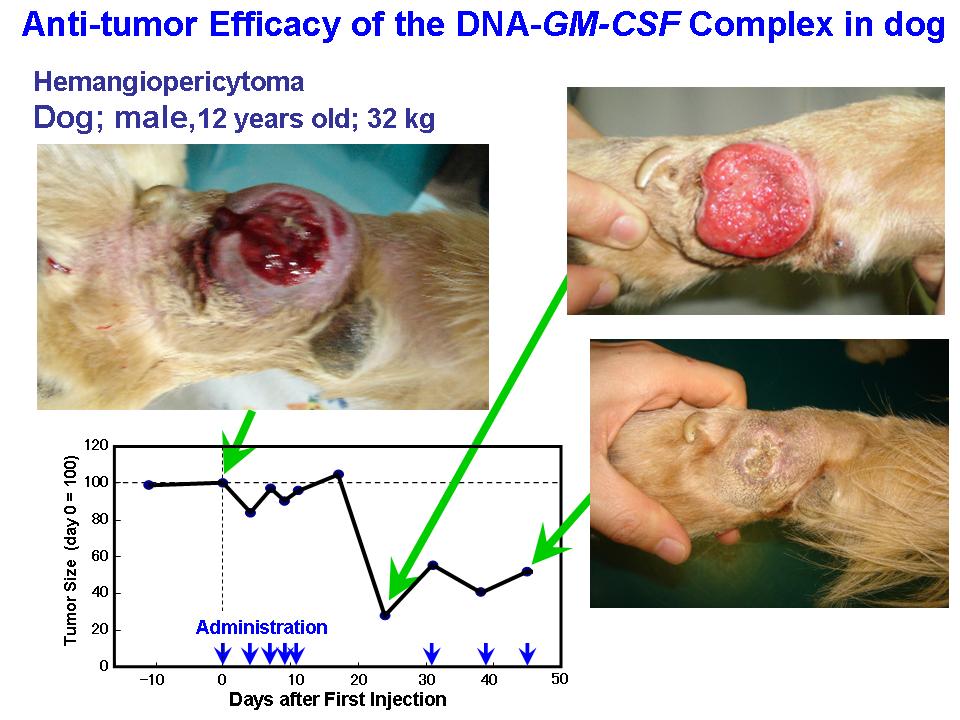
- 2-3. Antitumor Effect of the Synthetic Gene Delivery Systems
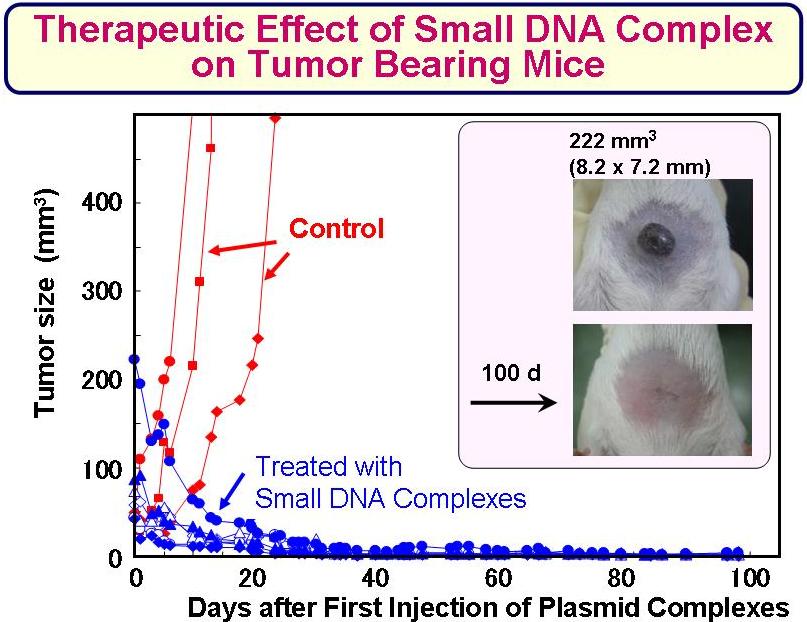
<Animal Clinical Study>
Small complex was prepared with DNA coding GM-CSF, a immuno-stimulating cytokine. Clinical effect of the DNA complex was examined on primary tumor in dogs, and cats. Those synthetic gene delivery systems showed remarkable anti-tumor efficacy on the animals.
- 2-4. Gene Medicine with Higher Anti-Tumor Efficacy
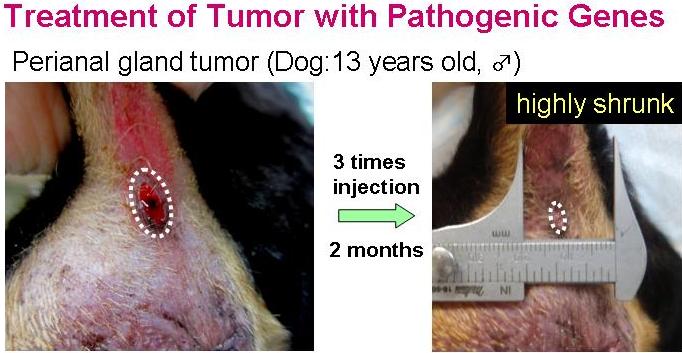
Transfection of the tumor-bearing mice with these pathogenic genes showed an evident anti-tumor activity. Co-transfection of the cytokine-genes, such as GM-CSF or IL-2, with the pathogenic antigen-gene showed highly superior anti-tumor therapeutic effect.
<Animal Clinical Study>
Animal clinical study on primary tumor-bearing dogs is now in progress in Japan and China. Evident suppression of the tumor growth was already confirmed in some cases.
Return to top